You will be asked to assign oxidation numbers to elements in a. The end outcome is the respond to the problem of the larger number separated by the smaller sized number.
 Oxidation Numbers Worksheet Answers Elegant Assigning Oxidation
Oxidation Numbers Worksheet Answers Elegant Assigning Oxidation Brent white created date.
Oxidation numbers worksheet. H 3aso 3 h. This method relates the number of electrons transferred to change in oxidation number. Use the rules for assigning oxidation numbers to determine the oxidation number assigned to each element in each of the given chemical formulas.
Ptcl 6 2 c. The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion. This quiz and worksheet will help you check your understanding of oxidation numbers and how to assign them to atoms in molecules.
Worksheet 25 oxidationreduction reactions oxidation number rules. Showing top 8 worksheets in the category oxidation. Mno 4 m.
Some of the worksheets displayed are work oxidation numbers name work 25 work assigning oxidation numbers chapter 20 work redox oxidation number exercise oxidation reduction handout work 1 determination of oxidation number or valence work 7. Elements have an oxidation number of 0 group i and ii in addition to the elemental oxidation state of 0 group i has an oxidation state of 1 and group ii has an oxidation state of 2. Sbf 6 i.
The oxidation number of fluorine in a compound is always 1. The oxidation number of a monatomic ion equals the charge of the ion. The oxidation number of a free element is always 0.
Cl 2 cl 16. Rules for assigning oxidation numbers the oxidation number of any uncombined element is 0 the oxidation number of a monatomic ion equals the charge on the ion. Balance the atom undergoing redox changes if necessary.
Formula element and oxidation number formula element and oxidation number 1. Some of the worksheets displayed are work 25 work oxidation numbers name work 7 oxidation number exercise redox practice work academic resource center chapter 20 work redox work assigning oxidation numbers. For example the oxidation number of na is 1.
Al 2o 3 f. Ptcl 4 2 n. Microsoft word 14 04 oxidation numbers worksheetdoc author.
Identify the changes in oxidation states and write the oxidation half reaction and the reduction half reaction and for each half reaction. Oxidation numbers worksheet directions. Na 2 o 2 na o 2.
Give oxidation numbers for the underlined atoms in these molecules and ions. The atoms in he and n 2 for example have oxidation numbers of 0. The usual oxidation number of hydrogen is 1.
The oxidation number of n 3 is 3. Showing top 8 worksheets in the category oxidation numbers. It consists of the following steps.
Hydrogen usually 1 except when bonded to group i or group ii when it forms hydrides 1.
 Redox Oxidation Numbers Practice Worksheet
Redox Oxidation Numbers Practice Worksheet 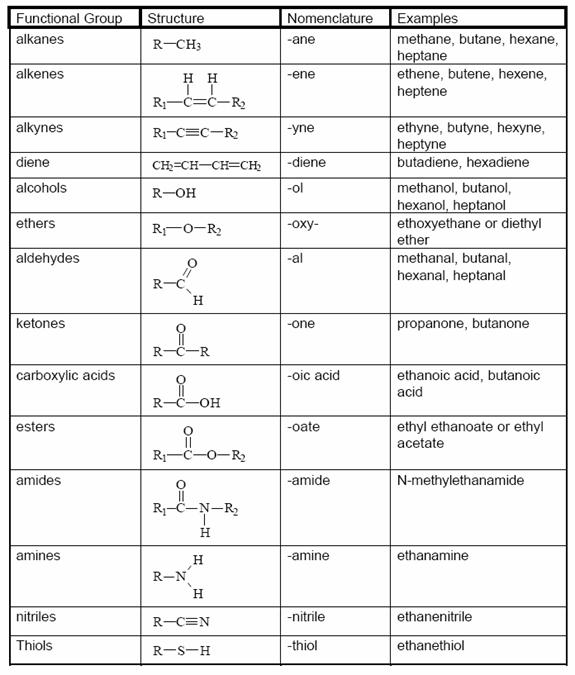 Assigning Oxidation Numbers Worksheet Answer
Assigning Oxidation Numbers Worksheet Answer 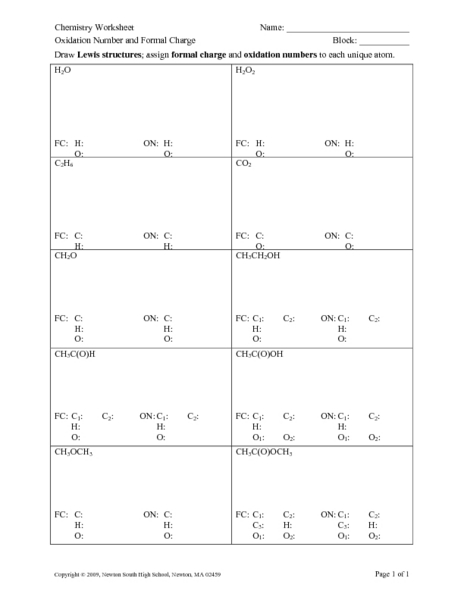 Chemistry Worksheet Oxidation Number And Formal Charge Worksheet For
Chemistry Worksheet Oxidation Number And Formal Charge Worksheet For  Oxidation Number And Redox Worksheet By Helenlochead Teaching
Oxidation Number And Redox Worksheet By Helenlochead Teaching 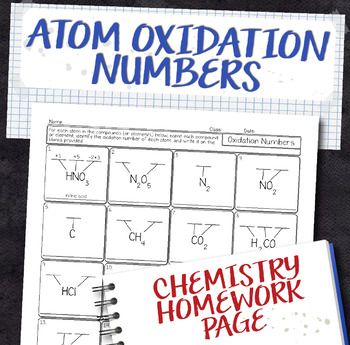 Oxidation Numbers Within A Compound Chemistry Homework Worksheet
Oxidation Numbers Within A Compound Chemistry Homework Worksheet  Oxidation Numbers Worksheet Answers Inspirational Collection Of
Oxidation Numbers Worksheet Answers Inspirational Collection Of 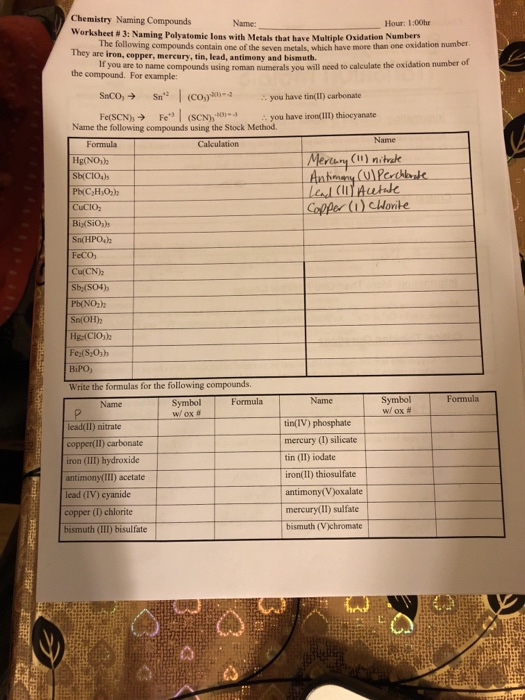 Solved Chemistry Naming Compounds Worksheet 3 Naming P
Solved Chemistry Naming Compounds Worksheet 3 Naming P
0 comments